Your Benzene ring carbon hybridization images are ready in this website. Benzene ring carbon hybridization are a topic that is being searched for and liked by netizens now. You can Get the Benzene ring carbon hybridization files here. Get all free photos and vectors.
If you’re looking for benzene ring carbon hybridization images information linked to the benzene ring carbon hybridization interest, you have visit the right blog. Our site frequently provides you with suggestions for refferencing the highest quality video and image content, please kindly surf and locate more informative video articles and graphics that match your interests.
Benzene Ring Carbon Hybridization. Give the hybridization of a carbon in benzene. Benzene Molecular Geometry And Bond Angles. Ad Super Angebote für Carbon Ring hier im Preisvergleich. Kotgiregeeta9959 kotgiregeeta9959 04062018 Chemistry Secondary School answered Structure of benzene write by hybridization of each carbon 2 See answers.
 What Is A Benzene Ring Natural Sciences 2022 From eng.sciencedevices.com
What Is A Benzene Ring Natural Sciences 2022 From eng.sciencedevices.com
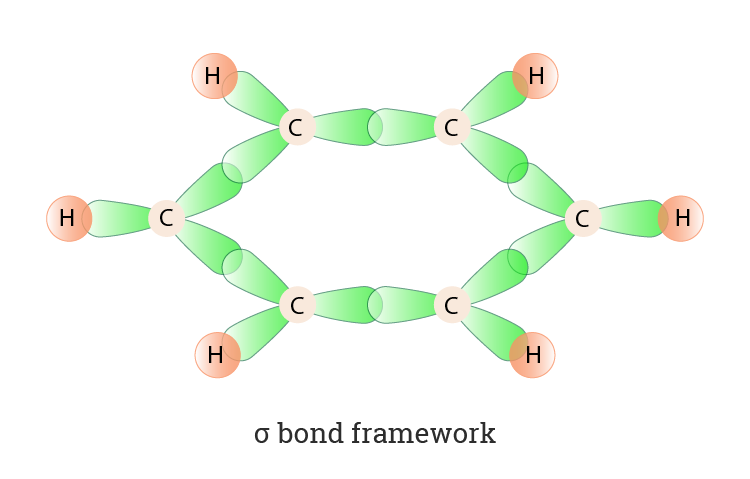
The next diagram shows the sigma bonds. The difference in benzene is that each carbon atom is joined to two other similar carbon atoms instead of just one. There are many derivatives of benzene. The sp2 hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane. The carbon atoms in the benzene ring are arranged in a trigonal planar geometry. A sp B sp2 C sp3 D sp3d E sp3d2.
This hybridization is a must to achieve the bond angle 120 which is.
The difference in benzene is that each carbon atom is joined to two other similar carbon atoms instead of just one. The sp2 hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane. A sp B sp2 C sp3 D sp3d E sp3d2. The carbon atoms in the benzene ring are arranged in a trigonal planar geometry. In benzene what is the hybridization on each carbon atom. Asked Jun 29 2017 in Chemistry by Daftaft.
 Source: shutterstock.com
Source: shutterstock.com
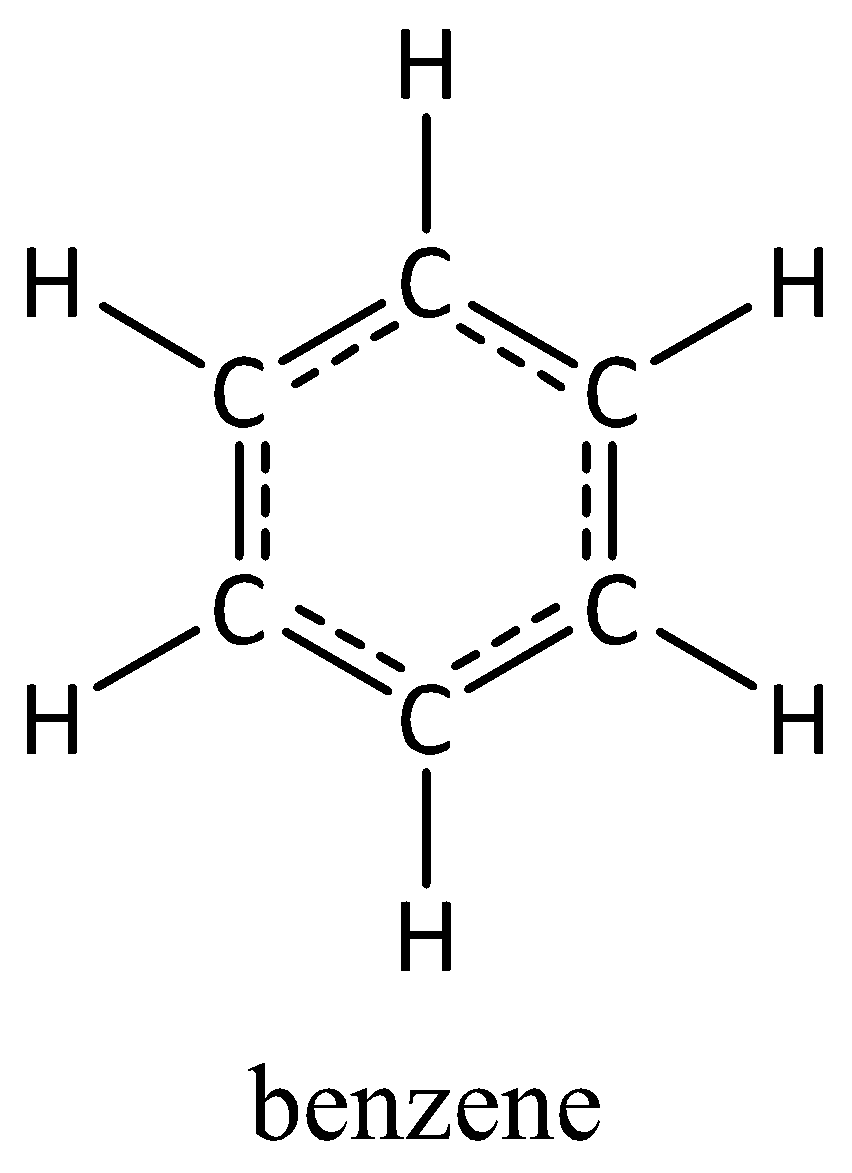
Because experimental data shows that the benzene molecule is planar that all carbon atoms bond to three other atoms and that all bond angles are 120 the benzene molecule must possess sp 2 hybridization. Benzene is an aromatic carbon compound containing alternative double bonds between the carbon atomsEach carbon is connected to two other carbon atoms of the ring and one hydrogen atom that lies outside the ring. In benzene the structure is in a form of a hexagon that alternate single and double bonds. Replacement of one of the hydrogen atoms with. Benzene is a combination of carbon and hydrogen atoms.
 Source: researchgate.net
Source: researchgate.net
During the hybridization process each carbon atom forms different bonds with two other similar carbon atoms instead of just one. The concept of hybridization comes from the idea of mixing pure atomic orbitals into hybrid orbitals to have efficient overlaps while bonding. Große Auswahl an Carbon Ring. Asked Jun 29 2017 in Chemistry by Daftaft. Each carbon atom now looks like the diagram on the right.
 Source: chemistrystudent.com
Source: chemistrystudent.com
Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore the hybridization is sp2. The next diagram shows the sigma bonds. Große Auswahl an Carbon Ring. Hybridization of carbon in benzene ring. This hybridization is a must to achieve the bond angle 120 which is found in benzene rings.
 Source: researchgate.net
Source: researchgate.net
The hydrogen atoms can be replaced by many different substituents. Now the other 6 sigma bonds come from the carbon to carbon bonds. During the hybridization process each carbon atom forms different bonds with two other similar carbon atoms instead of just one. Each carbon atom uses the sp 2 hybrids to form sigma bonds with two other carbons and one hydrogen atom. In benzene what is the hybridization on each carbon atom.
 Source: byjus.com
Source: byjus.com
Benzene is a planar aromatic ring and has many representations. In benzene what is the hybridization on each carbon atom. What is the hybridization of each carbon atom in benzene C6H6. The hybridization is sp 2 type. Answered Jun 26 2017 by Common.
 Source: researchgate.net
Source: researchgate.net
The concept of hybridization comes from the idea of mixing pure atomic orbitals into hybrid orbitals to have efficient overlaps while bonding. Answered Jun 26 2017 by Common. In other words an oxygen 2p orbital overlaps more effectively with the carbon 2p orbitals of the ring than an oxygen sp3 or-. It has an sp 2 hybridization. Since it has 3 σ bonds it has to be s p 2 hybridization.
 Source: angelo.edu
Source: angelo.edu
A sp B sp2 C sp3 D sp3d E sp3d2. The hybridization is sp 2 type. The benzene molecule comprises six carbon atoms joined in a ring with one hydrogen atom attached to each. What is the hybridization of carbon in benzene and cyclohexane respectively. The hybridization involved in the six carbon atoms of benzene is.
 Source: researchgate.net
Source: researchgate.net
Asked Jun 26 2017 in Chemistry by DarkFlame. Because experimental data shows that the benzene molecule is planar that all carbon atoms bond to three other atoms and that all bond angles are 120 the benzene molecule must possess sp 2 hybridization. The concept of hybridization comes from the idea of mixing pure atomic orbitals into hybrid orbitals to have efficient overlaps while bonding. What is the hybridization of all the carbon atoms in benzene c6h6. The hydrogen atoms can be replaced by many different substituents.
 Source: in.pinterest.com
Source: in.pinterest.com
This hybridization is a must to achieve the bond angle 120 which is. Regardless of whether we draw the Kekulé structure or the delocalized representation the structure is a ring containing carbon atoms that each had formed their first bond in three directions. Since it contains both Carbon and Hydrogen atoms we classify benzene as a hydrocarbon. The hydrogen atoms can be replaced by many different substituents. The sp2 hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane.
 Source: vedantu.com
Source: vedantu.com
Ad Super Angebote für Carbon Ring hier im Preisvergleich. In benzene each Carbon atom has 3 σ bonds and 1 π bond. There are many derivatives of benzene. 750 CHAPTER 16 THE CHEMISTRY OF BENZENE AND ITS DERIVATIVES This hybridization allows one of its electron pairs to occupy a 2p orbital which has the same size shape and orientation as the carbon 2p orbitals of the ring. This hybridization is a must to achieve the bond angle 120 which is.
 Source: in.pinterest.com
Source: in.pinterest.com
Hybridization of carbon in benzene ring. In benzene the structure is in a form of a hexagon that alternate single and double bonds. Each carbon atom now looks like the diagram on the right. The carbon atoms in the benzene ring are arranged in a trigonal planar geometry. Replacement of one of the hydrogen atoms with.
 Source: chemistryfromscratch.org
Source: chemistryfromscratch.org
Replacement of one of the hydrogen atoms with. This hybridization is a must to achieve the bond angle 120 which is. This hybridization is a must to achieve the bond angle 120 which is found in benzene rings. A sp B sp2 C sp3 D sp3d E sp3d2. Structure of benzene write by hybridization of each carbon Get the answers you need now.
 Source: youtube.com
Source: youtube.com
Since it contains both Carbon and Hydrogen atoms we classify benzene as a hydrocarbon. Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore the hybridization is sp2. Since it contains both Carbon and Hydrogen atoms we classify benzene as a hydrocarbon. With sp 2 hybridization each carbon atom has an unhybridized atomic p orbital associated with it. Give the hybridization of a carbon in benzene.
 Source: twitter.com
Source: twitter.com
This hybridization is a must to achieve the bond angle 120 which is found in benzene rings. What is the hybridization of the carbon atoms in benzene c6h6. This hybridization is a must to achieve the bond angle 120 which is found in benzene rings. Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore the hybridization is sp2. Answered Jun 29 2017 by SunVisitor.
 Source: chemistrystudent.com
Source: chemistrystudent.com
5 The hybridization of carbon atom in the benzene ring is Sp spd sp. In benzene what is the hybridization on each carbon atom. Ad Super Angebote für Carbon Ring hier im Preisvergleich. This hybridization is a must to achieve the bond angle 120 which is. The hybridization involved in the six carbon atoms of benzene is.
 Source: eng.sciencedevices.com
Source: eng.sciencedevices.com
The sp2 hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane. Since there are 6 carbon atoms each contributing 1 electron in the unhybridized p orbital then there are a total of six perpendicular 2p orbitals in each benzene ring. The carbon atoms in the benzene ring are arranged in a trigonal planar geometry. Because experimental data shows that the benzene molecule is planar that all carbon atoms bond to three other atoms and that all bond angles are 120 the benzene molecule must possess sp 2 hybridization. Große Auswahl an Carbon Ring.

Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore the hybridization is sp2. During the hybridization of benzene each carbon atom forms different bonds with two other similar carbon atoms instead of just one. Benzene contains a six-member carbon ring. Now the other 6 sigma bonds come from the carbon to carbon bonds. Hybridization of carbon in benzene ring.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore the hybridization is sp2. The sp2 hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane. 5 The hybridization of carbon atom in the benzene ring is Sp spd sp. Benzene is an aromatic carbon compound containing alternative double bonds between the carbon atomsEach carbon is connected to two other carbon atoms of the ring and one hydrogen atom that lies outside the ring. Answered Jun 26 2017 by Common.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title benzene ring carbon hybridization by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






