Your Carboxylic acid with benzene ring images are available. Carboxylic acid with benzene ring are a topic that is being searched for and liked by netizens now. You can Get the Carboxylic acid with benzene ring files here. Get all free photos.
If you’re looking for carboxylic acid with benzene ring images information connected with to the carboxylic acid with benzene ring topic, you have pay a visit to the right blog. Our site always gives you suggestions for seeing the highest quality video and picture content, please kindly search and find more enlightening video articles and images that fit your interests.
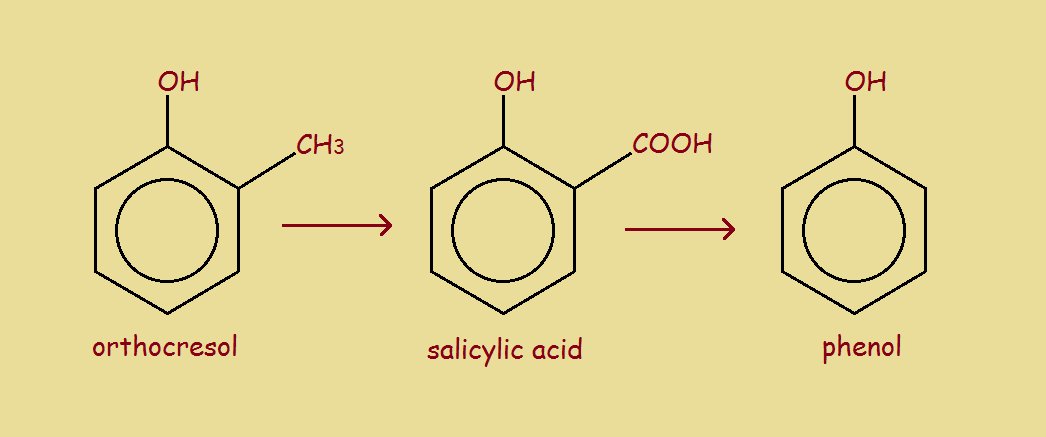
Carboxylic Acid With Benzene Ring. A carboxylic acid where the acid group is substituted to one carbon of a benzene ring. Carboxylic Acids Aqueous carboxylic acids are much acidic than alcohols and phenol. Explain why bromine reacts more readily with salicylic acid than with benzene. Then O-H bond gives electrons to the ring which increases polarity of O-H bond.
 Pin On Chemical Zoo From pinterest.com
Pin On Chemical Zoo From pinterest.com
Naming carboxylates Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the ic acid ending replaced by an ate ending. HC OH O CH3 COH O CH3CH2 COH O HO CCH2CH2CH3 O 6 Examples. Naming carboxylates Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the ic acid ending replaced by an ate ending. The common thing in phthalic acid and ox. Read More on This Topic chemical compound. The test which is used for the identific.
2H group on a ring are named using the suffix -carboxylic acid The -CO 2H carbon is not numbered in these cases Many non-systematic names.
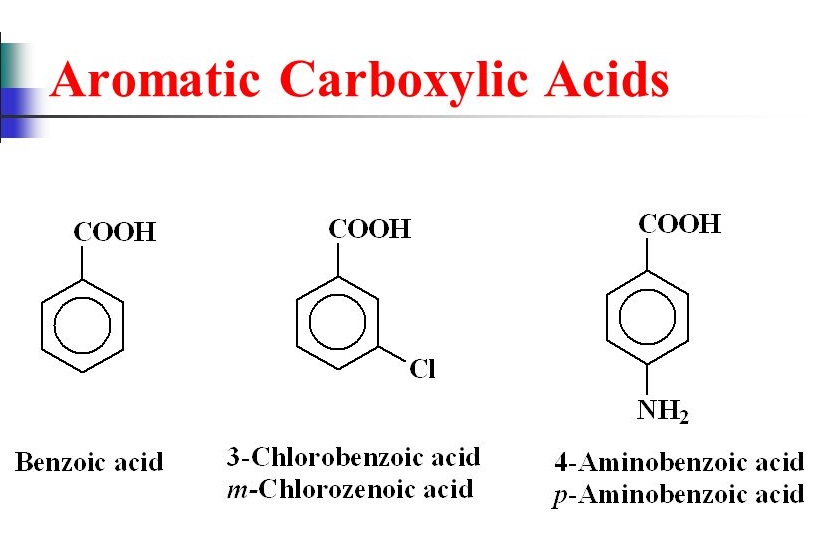
In the formation of Zwitter ions proton. Another example is 3-chlorocyclohexanecarboxylic acid which is shown below. Carboxylic Acids Aqueous carboxylic acids are much acidic than alcohols and phenol. Therefore it attracts electrons from benzene ring. In the above example tbutylbenzene does not contain a benzylic hydrogen and therefore doesnt undergo oxidation. The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid C 6 H 5 COOH.
 Source: pinterest.com
Source: pinterest.com
Therefore it attracts electrons from benzene ring. Read More on This Topic chemical compound. Naming Carboxylic Acids Name the following compounds. The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid C 6 H 5 COOH. Dry hydrogen chloride gas is used in some cases but these tend to involve aromatic esters ones where the carboxylic acid contains a benzene ring.
 Source: pinterest.com
Source: pinterest.com
Naming Carboxylic Acids Name the following compounds. A carboxylic acid where the acid group is substituted to one carbon of a benzene ring. The test which is used for the identific. Created by reacting an aromatic ring a carboxylic acid chloride and AlCl3 36. Organic hydrocarbons containing one or more benzene rings are called arenes.

The usual names of carboxylic acids make use of Greek letters not numbers to assign the placement of substituent groups in acids. HC OH O CH3 COH O CH3CH2 COH O HO CCH2CH2CH3 O 6 Examples. Attached to a benzene aromatic ring and are therefore named on the basis of being an aromatic compound. An example is chlorobenzene which is one of the halogenoarenes. A carboxylic acid where the acid group is substituted to one carbon of a benzene ring.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Then O-H bond gives electrons to the ring which increases polarity of O-H bond. C OH O Benzoic acid 5 Examples. Which acid is the manufacture of synthet. Therefore it attracts electrons from benzene ring. 2H group on a ring are named using the suffix -carboxylic acid The -CO 2H carbon is not numbered in these cases Many non-systematic names.
 Source: socratic.org
Source: socratic.org
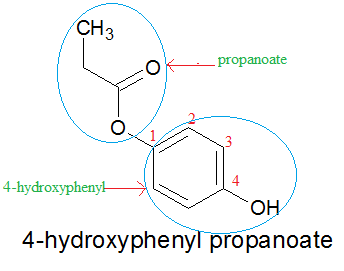
An carboxylic acid containing an additional hydroxy group -OH substistuted to another carbon atom than the acid group. This is followed by the name of the parent chain form the carboxylic acid part of the ester with an e remove and replaced with the ending oate. Carboxylic Acids Aqueous carboxylic acids are much acidic than alcohols and phenol. A carboxylic acid where the acid group is substituted to one carbon of a benzene ring. Which acid is the manufacture of synthet.

The chemistry of the reaction Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Friedel-Crafts Reaction Acyl chloride is used in place of alkyl chloride. The chemistry of the reaction Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid C6H5COOH. Dry hydrogen chloride gas is used in some cases but these tend to involve aromatic esters ones where the carboxylic acid contains a benzene ring.
 Source: pinterest.com
Source: pinterest.com
Friedel-Crafts Reaction Mechanism Step 1. An carboxylic acid containing an additional hydroxy group -OH substistuted to another carbon atom than the acid group. Dry hydrogen chloride gas is used in some cases but these tend to involve aromatic esters ones where the carboxylic acid contains a benzene ring. In the above example tbutylbenzene does not contain a benzylic hydrogen and therefore doesnt undergo oxidation. Naming Carboxylic Acids Name the following compounds.
 Source: pinterest.com
Source: pinterest.com
When carboxylic acids are heated with alcohols in the presence of a catalyst which is acid at such time esters are produced. Another example is 3-chlorocyclohexanecarboxylic acid which is shown below. In the above example tbutylbenzene does not contain a benzylic hydrogen and therefore doesnt undergo oxidation. This is true for both the IUPAC and Common nomenclature systems. Directly connected to a benzene ring are named after the parent compound benzoic acid.
 Source: quizlet.com
Source: quizlet.com
Under this system of nomenclature benzoic acid. If you draw a benzene ring with one group attached you have drawn a phenyl group. Friedel-Crafts Reaction Acyl chloride is used in place of alkyl chloride. Therefore it attracts electrons from benzene ring. CH3CHCH2 COH Br O CH3CHCH2 COH CH2CH2CH3 CH3 O COH O.
 Source: online-sciences.com
Source: online-sciences.com
Therefore it attracts electrons from benzene ring. Are carboxylic acid derivatives where the OH group is replaced by another group. An example is chlorobenzene which is one of the halogenoarenes. The catalyst is usually concentrated sulfuric acid. The product is a phenyl ketone that is less reactive than benzene.
 Source: pinterest.com
Source: pinterest.com
In your answer you should use appropriate technical terms spelledcorrectly. C OH O Benzoic acid 5 Examples. The acid with the carboxyl team attached directly to a benzene ring is called benzoic acid. The product is a phenyl ketone that is less reactive than benzene. Explain why bromine reacts more readily with salicylic acid than with benzene.
 Source: pinterest.com
Source: pinterest.com
Alkyl groups that contain benzylic hydrogens hydrogen s on a carbon α to a benzene ringundergo oxidation to acids with strong oxidizing agents. Carboxylic acid - Aromatic acids Britannica Aromatic acids Aromatic acids include compounds that contain a COOH group bonded to an aromatic ring. An example is chlorobenzene which is one of the halogenoarenes. In a substituted aromatic carboxylic compound the name of the compound is derived from benzoic acid. When more NO 2 groups connect to the ring polarity of O-H bond more increases.
 Source: pinterest.com
Source: pinterest.com
Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with an a t e ending. Friedel-Crafts Reaction Acyl chloride is used in place of alkyl chloride. Attached to a benzene aromatic ring and are therefore named on the basis of being an aromatic compound. Naming carboxylates Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the ic acid ending replaced by an ate ending. HC OH O CH3 COH O CH3CH2 COH O HO CCH2CH2CH3 O 6 Examples.
 Source: in.pinterest.com
Source: in.pinterest.com
The simplest aromatic acid is benzoic acid. 2H group on a ring are named using the suffix -carboxylic acid The -CO 2H carbon is not numbered in these cases Many non-systematic names. These letters describe the position of the carbon atom in relationship to the carboxyl carbon atom. Naming carboxylates Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the ic acid ending replaced by an ate ending. Carboxylic acid - Aromatic acids Britannica Aromatic acids Aromatic acids include compounds that contain a COOH group bonded to an aromatic ring.
 Source: pinterest.com
Source: pinterest.com
The common names of carboxylic acids use Greek letters α β γ δ and so forth not numbers to designate the position of substituent groups in acids. The simplest aromatic acid is benzoic acid. In the above example tbutylbenzene does not contain a benzylic hydrogen and therefore doesnt undergo oxidation. The aromatic ring carbon that carries the carboxyl group is marked by the position number 1. Another example is 3-chlorocyclohexanecarboxylic acid which is shown below.
 Source: quirkyscience.com
Source: quirkyscience.com
Then O-H bond gives electrons to the ring which increases polarity of O-H bond. 3 Bromine reacts more readily with salicylic acid than with benzene because the lone pair of electrons on the OH group in salicylic acid is partially delocalised into the ring. Are carboxylic acid derivatives where the OH group is replaced by another group. Explain why bromine reacts more readily with salicylic acid than with benzene. In the formation of Zwitter ions proton.
 Source: chem.ucla.edu
Source: chem.ucla.edu
The aromatic ring carbon that carries the carboxyl group is marked by the position number 1. Another example is 3-chlorocyclohexanecarboxylic acid which is shown below. Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with an a t e ending. Friedel-Crafts Reaction Acyl chloride is used in place of alkyl chloride. Naming Carboxylic Acids Name the following compounds.
 Source: pinterest.com
Source: pinterest.com
The test which is used for the identific. Organic hydrocarbons containing one or more benzene rings are called arenes. In your answer you should use appropriate technical terms spelledcorrectly. Directly connected to a benzene ring are named after the parent compound benzoic acid. The common names of carboxylic acids use Greek letters α β γ δ and so forth not numbers to designate the position of substituent groups in acids.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title carboxylic acid with benzene ring by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






